Last month the agency submitted its proposed rule on front-of-pack nutrition labeling to the Office of Management and Budget after nearly two years of examining different schemes and even longer mulling industry feedback about the idea, which it floated in 2010 following public outrage about alleged abuses of the FOP Smart Choices labeling program.
The main goal of mandatory front-of-pack labeling is a desire to help consumers make healthier choices by highlighting select information from the Nutrition Facts and Ingredients decks and potentially providing context to help shoppers interpret how a product’s saturated fat, sugar and sodium levels might impact their wellbeing.
But how might FOP impact food and beverage manufacturers’ approach to product innovation, renovation and marketing?
In this episode of FoodNavigator-USA’s Soup-To-Nuts podcast, legislators, including Sen. Bernie Sanders, share why front-of-pack labeling is important, and Michelle Briffett, a principal with the global consultancy Roland Berger, breaks down how different front-of-pack labeling schemes could influence consumers as well as next steps for manufacturers, including potentially reformulating or repositioning products.
Sanders chastises FDA for ‘unacceptable’ missed deadlines
After years of lobbying and waiting for FDA to draft and share a proposed front-of-pack nutrition labeling scheme, US legislators on both sides of the aisle are frustrated by multiple missed deadlines and what they consider unnecessary delays.
Last week at a hearing hosted by the Senate Committee on Health, Education, Labor and Pensions committee, independent Vermont Sen. Bernie Sanders turned up the heat on the agency to mandate front-of-pack nutrition labeling as part of a broader effort to “reduce the diabetes and obesity epidemics in America and take on the greed of the food and beverage industry.”
Subscribe to FoodNavigator-USA's podcasts and catch up on past episodes
Never miss an episode of FoodNavigator-USA’s Soup-To-Nuts podcast or our recently launched Founders’ Fundamentals podcast – subscribe today.
And if you haven’t already, listen to these recent episodes:
- What will it take for the US hummus market to break $1 billion annually?
- Where are consumers most interested in sugar reduction?
- Consumers increasing turn to food for ‘emotional wellness’
- What does USDA’s stricter definition for ‘pasture-raised’ chicken mean for industry?
- What is the business proposition for 100% grass fed dairy?
He attributed the extent of diabetes and obesity in the US in part to Congress and the FDA “allowing” corporations to profit from “enticing children and adults to consume ultra processed food and beverages loaded up with sugar, salt and saturated fat.”
He also asked what FDA has done to address these epidemics and why it has missed multiple self-imposed deadlines for proposing a front-of-pack labeling scheme following a recommendation in 2010 by the National Academy of Medicine.
“We are not waiting to act where the evidence is clear. In addition to front-of-pack nutrition labeling, FDA is working to reduce sodium across the food supply, update the ‘healthy’ claim on food packages and strengthen our chemical safety review program.”
FDA Commissioner Robert Califf
At the hearing, FDA Commissioner Robert Califf agreed that mandating front-of-pack labeling of key nutrition information could be a “landmark” policy and dramatically improve the health of the nation. But disagreed that FDA is dragging its feet to enact FOP labeling. Rather, he called on Congress for addition help to move the initiative over the finish line, along with other complementary initiatives that he said would improve the healthfulness of the US food supply.
“We are not waiting to act where the evidence is clear. In addition to front-of-pack nutrition labeling, FDA is working to reduce sodium across the food supply, update the ‘healthy’ claim on food packages and strengthen our chemical safety review program,” all of which require additional funding and experts to execute, he said.
Why is front-of-pack nutrition labeling important?
Califf’s comment about the need for a multi-prong approach to improving the diet and health of Americans is shared by many stakeholders, but it also begs the question – why are public health advocates, legislators and FDA placing so much emphasis and dedicating so many resources to front-of-pack labeling?
The answer, according to Roland Berger Principal Michelle Briffett, may lie in the demonstrated impact in Chile and other countries that already mandate front-of-pack nutrition labels like those which FDA is considering.
She explained within a year of Chile implementing front-of-pack nutrition labeling alongside other nutrition initiatives, households reduced their calories 3.5% and consumers gravitated to products without warnings.
Chile implemented a labeling scheme that doubled as a warning system – cautioning consumers when products had high levels of calories, saturated fat, added sugars and other nutrients of concern. But this is not the only option. Other common front-of-pack labeling systems include “repeated systems,” that simply repeat on the front of pack key information from the back of the pack, and a more complicated spectrum system that gives products an overall score based on all their nutrition information.
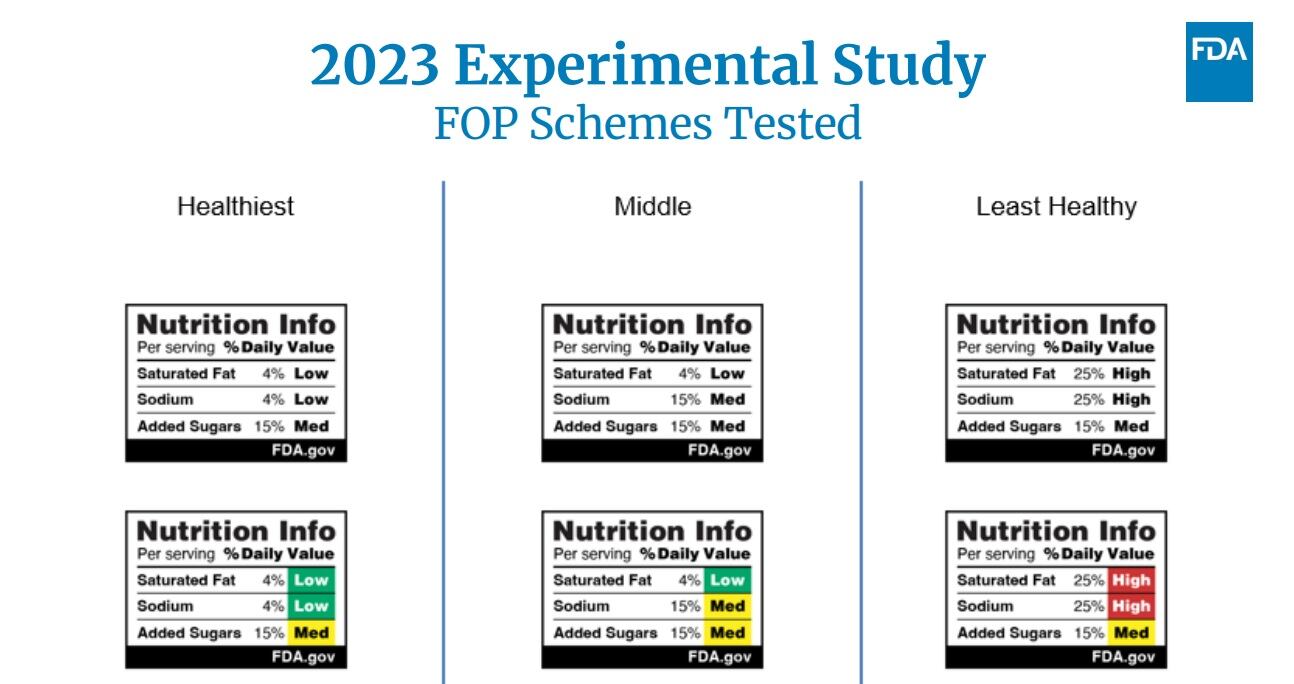
As Briffett mentioned, all three FOP schemes move to the front panel nutrition information that already is available elsewhere on the package – so why is it needed? How hard is it for consumers to pick up a package, flip it over and read the information?
Pretty hard, according to Briffett, who cited research conducted by FDA that found most consumers do not understand the nutrition information on packages or how it impacts their health.
“The front of package schemes have come up as a way to really circumvent some of those challenges and give people fast and easy access to information of things that they really need to know about products to make informed decisions in a matter of seconds,” she explained.
FDA could propose a repeated or warning system
Of the three most popular FOP labeling schemes, Briffett said FDA is most likely to propose either the ‘repeated system’ or a warning system to highlight the amount of saturated fat, sodium and sugar in products. She notes that both have pros and cons.
A repeated system is the easiest to approve as it simply highlights information that is already available and does not pass judgement. But it is not as easy for consumers.
Research suggests the warning system is more impactful, but it could be harder to approve, she explained.
Now is the time to reformulate, innovate, rethink marketing
According to Briffett, either option would have major strategic implications for many food and beverage brands, including potentially reformulating products, innovating complementary better-for-you options to sit alongside indulgent products or adjusting serving sizes to avoid triggering a “warning” on the front of pack.
No matter which front-of-pack scheme is proposed, Briffett argues it will create “winners and losers” across different categories, which would not only pose a challenge for brands but also could pose a challenge to FDA finalizing an FOP labeling mandate.
Brands that want to pushback will have a chance to do so – as will stakeholders that support any scheme FDA puts forward. The agency is working on a proposed rule – not a final rule – for front-of-pack labeling, which means there will be opportunity to submit comments.
Briffett adds there also will be time to comply with the final rule when it is passed. She notes that companies usually have more than a year to make mandatory label changes and smaller manufacturers often have extra time to implement changes. And, of course, there will be time between the proposed rule, gathering and analyzing comments and finalizing the rule.
Briffett notes that while brands have time, they may want to think about reformulation and innovation now – as many macro-trends align with FDA’s labeling efforts, including consumer demand for better-for-you products with lower sugar, sodium and saturated fat.




