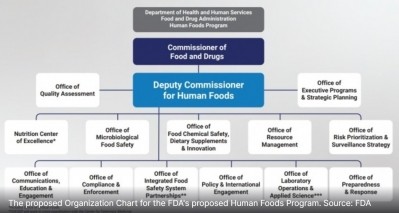
FDA Human Foods Program launch may not come until next fall, but ‘going quite well’

When Jones stepped into his role last fall it was with a laser focus to create a Human Foods Program that will bring together 12 offices and one center under a single decision-maker for food related issues.
Two months into his tenure, Jones estimated the new program would be up and running by June 2024, but now, nearly six months since he took the helm, Jones says he is “reasonably confident” the program will be in place and operational by the end of this fiscal year, which for FDA is Sept. 30.
The agency submitted the proposed reorganization package in December 2023 for a formal external review process required for all federal organizations, and while Jones said the process so far has “gone quite well,” he added that “FDA, or HHS for that matter, doesn’t control the progress. So, it is very hard to predict when that process is going to come to closure.”
Still, he projected optimism as he explained “the kinds of questions that we are getting are questions that we are easily able to answer, which means to me that we’ve got a package that holds together and it is explained pretty well.”
Jones' position is cornerstone of new Human Foods Program
Until then, the only part of the reorganization that is in place is Jones’ position as a single leader to whom the directors of the offices that will eventually be joined under the Human Foods Program are already reporting.
This may be only a small part of the overall plan, but as Jones explained it is a pivotal change that should lay a foundation for a more cohesive approach to food policy and provide operational clarity to key stakeholders compared to the prior system which empowered multiple leaders and which caused division and strife within FDA’s nutrition programs.
“There has been a lot of focus on the interplay between the two offices at the top, but I actually think whenever you have more than one decisionmaker, in any context, that goes all the way through the organization. People are trying to figure out where there issues ultimately are going to get decided, who is going to decide and how to manage it in a way to make sure that they get the outcome they are looking for,” he said.
“Now, it is pretty clear who the decisionmaker is in the long run. People better understand now how I make decisions and bout how I get the framework that I want for them to use to make decisions,” he said.
Nutrition will have ‘much greater prominence in the FDA’
Given this clarity, Jones is moving forward with a clearly communicated agenda for the new program, which he described as “pretty ambitious and exciting” and which he said will give nutrition “much greater prominence in the FDA.”
From a nutrition perspective, the agenda includes a combination of regulatory, programmatic and voluntary initiatives focused on reducing sodium and added sugar in the food supply, a new definition of healthy, updated front-of-pack labeling and “evidence-based approach” to better understanding the impact of ultra processed food on health. The agenda also includes initiatives to promote food chemical safety and preventing food borne illness.
As Jones explained, some of the nutrition initiatives are advancing more quickly and some remain in early stages.
New sodium reduction targets coming this summer
For example, FDA anticipates “big news coming later on this year” around the next steps in the current voluntary sodium reduction program.
Jones explained that data shows the industry is on track to reduce sodium by 12% by next month, and as such he anticipates “having another step-down goal that we hope to have in the public domain by this summer.”
The agency currently is measuring sodium reduction in 160 food categories and while there is a one year lag in the data that is reported, Jones said “preliminary results are quite encouraging.”
He added that the voluntary nature of the program is working well, which he acknowledged is not always true.
“My experience is [voluntary programs] don’t always work, but when they do work, you ride them as long as you can. It allows for ultimate flexibility for those who are trying to meet the goal, and you get more optimal outcomes if you can do something like this voluntarily,” he explained.
Added sugar reduction efforts are ‘not obvious’
Simultaneous efforts to reduce added sugar in the US food supply are not as far along as those to reduce sodium, and Jones admits “this is an area where we are not exactly sure what kind of approach we want to take.”
The agency recently hosted a public meeting to gather feedback from stakeholders and Jones said there will be “at least one more opportunity for public comment before we ultimately decide what path to go down.”
Definition of healthy and front-of-pack labeling could come within months
Two other long-promised and high profile initiatives – an updated definition of healthy and front-of-pack labeling to help consumers understand nutrition in products – could be brought forward as soon as this spring or early summer, Jones said.
Jones said he is “quite confident” that FDA will share this spring or early summer an updated standard for when a manufacturer can call a product healthy. The update – the first in 20 years – will also include a logo that could allow consumers to readily recognize if a food is considered healthy by the government.
“That kind of tool can be an incredibly effective way to communicate to consumers about the characteristics of a product,” he said, adding that FDA has conducted “a fair amount of consumer testing” on the logos it is considering.
In addition, Jones said, FDA is “in the midst right now of actually writing up the proposal” for front-of-pack labeling, which he anticipates will further help consumers understand the healthfulness of products.
‘We feel like there needs to be more research’ on ultra processed foods
An emerging area on FDA’s radar is the growing debate around the role of ultra processed foods in the American diet.
Recognizing that UPF has become a flashpoint in the food and nutrition communities, Jones said that FDA is working with the National Institutes of Health, other federal and regulatory agencies, the executive branch and research community to “figure out what are the questions we need to answer before we can have an evidence-based approach on ultra processed food.”
He explained that FDA takes action when it has evidence, but currently this is “an area where we feel like there needs to be more research."
He added that FDA is closely watching the 2025 Dietary Guidelines Advisory Committee, which is looking into UPF.
Ultimately, Jones said, the yet-to-be-finalized Human Foods Program has a “pretty ambitious nutrition portfolio” and an ambitious goal to help turn around nutrition, which he said “is going in the wrong direction in this county” and negatively impacting millions of Americans.